what is the valence electron configuration for the arsenic atom|Arsenic valence electrons : Manila The valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron . Tingnan ang higit pa Analiza la evolución y el estado actual del tipo de cambio de wones surcoreanos/pesos chilenos y recibe, sin coste, alertas por correo electrónico sobre el estado del tipo de cambio. . 20.528,34000 CLP: 40000 KRW: 27.371,12000 CLP: 50000 KRW: 34.213,90000 CLP: Tipo de cambio Peso chileno / Won surcoreano; 1 CLP: 1,46139 KRW: 5 CLP: .
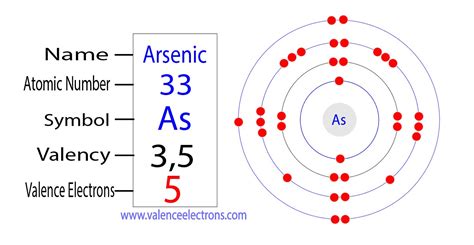
what is the valence electron configuration for the arsenic atom,The valence electrons are the total number of electrons in the last orbit(shell). The total number of electrons in the last shell after the electron configuration of arsenicis called the valence electrons of arsenic. The valence electrons determine the properties of the element and participate in the . Tingnan ang higit paThe valence electrons have to be determined by following a few steps. The electron configuration is one of them. It is not possible to determine the valence electron . Tingnan ang higit paThe elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). The number of unpaired electrons in the last orbit of an . Tingnan ang higit pa
Arsenic is in Group 15 (also called 5A) and therefore As has five valence electrons (not that this method doesn’t work for the Transition Metals). You can also look at the electron.
A step-by-step description of how to write the electron configuration for Arsenic (As and the As 3- ion).In order to write the As electron configuration we . Its shorthand electron configuration is: [Ar]3d104s24p3. Explanation: As is the chemical symbol for the element arsenic. Its atomic number is 33, which is the number of protons in the nuclei of its atoms. .

Answer link. Arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. It's outermost shell (4s and 4p) has 5 electrons, these are the valence electrons. Methods. We can write the valence electrons of arsenic using two different methods: #1 Using periodic table. #2 Using electron configuration. Let’s break down each method in detail. Using periodic table. Periodic table | Image: Learnool. Get the periodic table having the chemical elements marked on it as mentioned above. It has the representative symbol as, As and the atomic number 33. Flerovium Valence Electrons. Helium Valence Electrons. Plutonium Valence Electrons. Lithium Valence Electrons. Mercury Valence electrons. Americium Valence Electrons. Neptunium Valence Electrons. Oxygen Valence Electrons. Moscovium Valence . You can see in the electron configuration of arsenic (1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3) that the highest energy level is 4. And the total number of electrons present in this energy level is 2 + 3 = 5. So by knowing the electron configuration, we have found that the Arsenic has 5 valence electrons. Nitrogen Valence Electrons; Oxygen Valence Electrons; Arsenic Number of Valence Electrons. Valence electrons are the number of electrons that are present at the outermost shell of the atom or . The electronic configuration of arsenic, [Ar] 3d10 4s2 4p3, shows that there are 5 electrons in the outermost shell of arsenic. These electrons are located in the 4s and 4p orbitals of the N shell. The valence electrons of arsenic are the 5 electrons in the outermost shell, and they are involved in chemical bonding with other elements. 4.
Exercise 7.3.13 7.3. 13. Hund's rule states that the most stable arrangement of electrons (for a ground state electron configuration) has a filled valence shell of electrons. has three electrons per orbital, each with identical spins. has values greater than or equal to +1. has the maximum number of unpaired electrons, all with the same spin.what is the valence electron configuration for the arsenic atom Arsenic valence electrons The arsenic orbital diagram is a graphical representation of the electron configuration of the arsenic atom. This diagram shows how the electrons in the arsenic atom are arranged in different orbitals. Orbital is the .
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information (Figure 6.25): The number of the principal quantum shell, n, The letter that designates the orbital type (the subshell, l), and
Arsenic Electron Configuration: Chemical element, Arsenic has atomic number 33 and has the symbol “As”. Arsenic chemical occurs in many minerals like elements with a combination of Sulphur and metals. This is the pure elemental crystal. Arsenic comes in a metalloid category. This arsenic element has different allotropes .
1 Answer. Arsenic has 5 valence electrons. It's electron configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p3. It's outermost shell (4s and 4p) has 5 electrons, these are the valence electrons. Arsenic has 5 valence electrons. It's electron configuration is 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^3. It's outermost shell (4s and 4p) has 5 .The complete electron configuration of arsenic is written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. Arsenic have 5 valence electrons in the 4s and 4p subshells around the nucleus and the atomic number is 33 .The distribution of electrons is as 2 electrons in 1s subshell, 2 electrons in 2s subshell, 6 electrons in 2p subshell, 2 electrons in 3s, 6 .The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z .The configuration of these electrons follows from the principles of quantum mechanics. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number Z . The electron configuration of arsenic is [ Ar] 4s 2 3d 10 4p 3. In the above electron configuration, the highest energy level (4) is marked with green color. The 4 th energy level contains 4s and 4p subshells. There are 2 electrons in the 4s subshell and 3 electrons in the 4p subshell. So arsenic has a total of 2 + 3 = 5 valence electrons.

Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Arsenic .
Arsenic valence electrons Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Arsenic . So, lose two electrons, and when calcium loses two electrons, it becomes the calcium 2-plus cation. And then we can write the electron configuration really short, or it'll be really short because we know it's going to lose these two valence electrons . Electron Configuration of Arsenic: Follow the steps mentioned below to get the electron configuration of Arsenic. To write the electron configuration of arsenic, we should first know the total number of electrons present in an arsenic atom. The arsenic atom has a total of 33 electrons because its atomic number is 33 and it is a .what is the valence electron configuration for the arsenic atom Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to ..
When writing an electron configuration, you have to write serially. Barium ion(Ba 2+) electron configuration. The ground-state electron configuration of barium is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 6s 2. This electron configuration shows that the last shell of barium has two electrons. Therefore, the valence electrons of .For each of the following pairs of atoms or ions, state which you expect to have the larger radius. (a) Na or K (b) Cs or Cs+ (c) Rb+ or Kr (d) K or Ca (e) CI- or Ar. A possible excited state for the \mathrm {H} H atom has an electron in a 5 d 5d orbital. List all possible sets of quantum numbers n, \ell n,ℓ, and m_ {\ell} mℓ for this electron. Electron configurations describe where electrons are located around the nucleus of an atom. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has .
what is the valence electron configuration for the arsenic atom|Arsenic valence electrons
PH0 · What is the electron configuration for As?
PH1 · How to write the electron configuration for Arsenic (As and the As
PH2 · How to Find the Valence Electrons for Arsenic (As)?
PH3 · How to Find the Valence Electrons for Arsenic (As)
PH4 · How many valence electrons does Arsenic have?
PH5 · Electron Configuration for Arsenic (As, As3
PH6 · Arsenic valence electrons
PH7 · Arsenic Valence Electrons (And How to Find them?)
PH8 · Arsenic Valence Electrons
PH9 · Arsenic Electron Configuration (As) with Orbital Diagram